Children's Tumor Foundation
Innovative Patient Reported Registry Platform

Background
Established in 1978, Children’s Tumor Foundation was originally developed on the ground with the sole objective of collecting and collating information that could be used to inform, and develop treatments for neurofibromatosis (NF) type 1 and 2. Through their continued work and dedication over the years Children's Tumor Foundation has established itself as the foremost patient-led group in the world for NF1, NF2 and schwannomatosis.
Children's Tumor Foundation originally embarked on their registry journey in 2012. In 2019 the decision was made that their current platform did not meet their needs and optimally support expanding the global reach of the system, so they elected to find to an experienced registry provider to implement improvements and add new features with the view of developing an international flagship registry for NF.
What is NF and why a patient registry?
Neurofibromatosis (NF) affects 0.03% of people worldwide and refers to a group of genetic disorders which causes tumors to grow on nerve tissue, the condition can affect any part of the nervous system. The term NF is commonly used to represent three distinct disorders NF1, NF2 and schwannomatosis There is no known cure at present, but treatments and medications are used to manage symptoms, mitigate complications and give sufferers quality of life.
A patient registry was paramount for Children's Tumor Foundation to engage their patients and to accrue real world data (RWD) that will go towards to informing clinical guidelines, optimal treatment plans/regimes, clinical trial design and recruitment etc. NF patients need to be intrinsically involved in the registry platform to ensure sustainability of the system and engage them in the data collection process, for this reason it was decided to have patient reported data functionality (PRD), meaning that patients or their careers can access the platform and enter information on their condition. This data is essential to keep up to date with the patient perspective and experience, giving a comprehensive view of NF today.
The NF registry is a ground-breaking project, where the OpenApp team worked in collaboration with CTF to develop and support a platform that meets their requirements, as a secure and effective tool to empower NF patients and their caregivers. A dedicated registry is the most efficient way to raise awareness/advocate for NF, expand the NF community, and connect to help end NF.
Patient Registry Objectives
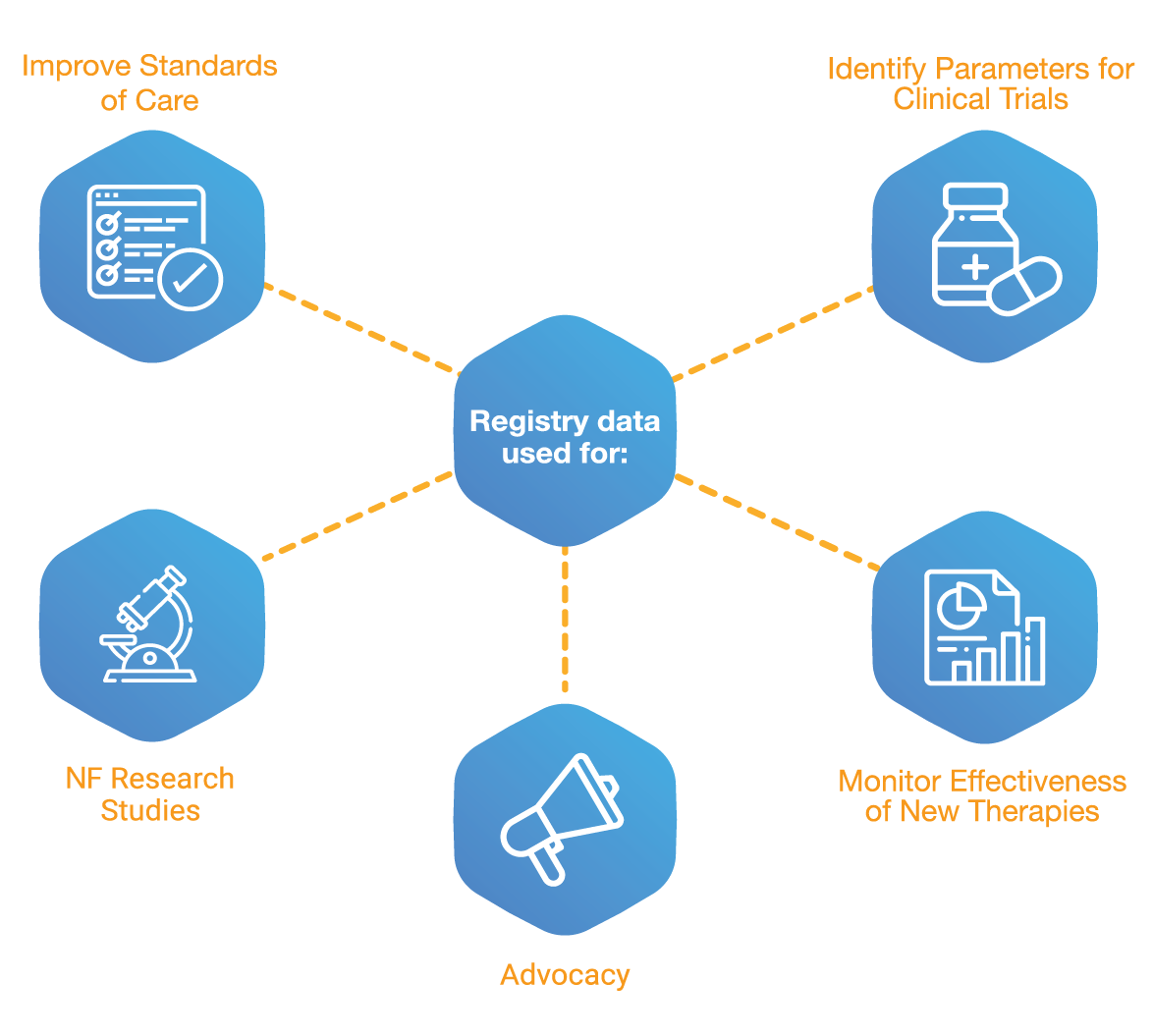
• One steadfast database to house all NF patients: There was a need to build a system with the technical capacity and robustness to house information on all NF patients located worldwide irrespective of their treatment plans.
• Communication bridge: The registry should further connect the patients to the patient organization, Children's Tumor Foundation, and connect them with the researchers which helps them to advance NF related research. The registry will give patients scope to report on their well-being and treatments based on endpoints and parameters outlined by Children's Tumor Foundation, producing high quality and usable data.
• Easy data entry: The registry must have a user-friendly interface where the patients or their representatives can seamlessly input their data without any obstacles. Visual representations of symptoms are to be used where appropriate for clarity.
• Patient Consent: Have a platform whereby patients consent to the data they are willing to share, including full control of the permissions on when, or if, you are to be contacted. Patients also have the facility to rescind consent at any time they see fit. All information is carefully protected in alignment with the strictest privacy protocols.
• Surveys and Forms: The registry must be capable of running customized, disease-specific surveys and forms that users can move through and complete smoothly.
• Information Hub: A platform capable of informing patients about new NF focused treatments and studies.
• An international approach: One of the main requirements for Children's Tumor Foundation was a platform that was viable for an international audience, with the capacity to house patients located around the world. This is achieved by having the technical capacity to extend the registry’s reach to a more diverse racial, ethnic, and geographic cohort of participants. This means the data collected was more representative of the entire patient population, improving the understanding of quality of life and key health markers of NF patients globally.
• Multilingual: Due to the international approach, Children's Tumor Foundation required a registry that supports multiple language interfaces, and needed a platform with the flexibility and capacity to add more languages as the global reach of the system expands.
• Patient Reported Data(PRD) Supported: The registry needs to cater to patients and/or their representatives entering data. Developing a system that patients could engage with and relay their experience through intuitive data entry was of utmost importance.
• Continuously Building a Cohort: Create a registry with ease of access and reduced barriers to entry for NF patients.
• Benchmarking: Patients should be able to see how their answers compare to the wider patient population.
Actions
Specific and comprehensive action was taken to create a patient entered data resource where patients, or their representatives, are always in control of their own information, and can participate in accelerating research and finding treatments for all forms of NF. Everything from the applied dataset to the acceptance criteria into the system has been oriented towards NF patients and their engagement.
Collaborative scoping with team of experts – Children's Tumor Foundation and OpenApp collaborated to finalize the required dataset and platform workflows for a patient reported system, advising on data gathering techniques and approaches, technical aspects of user experience and potential future platform functionalities and workflows for later phases. This was achieved through several workshops, where prototypes were shared and demonstrated by our business analysis, user experience and technical teams.
Data validation – The delivered registry has been configured in accordance with CTF requirements to make it easier for the patients to enter their data accurately. Business rules and edit checks were implemented to keep data accurate and mitigate any data entry errors, these validation rules ensure consistent data quality and completeness.
Privacy and data protection – The registry platform complies with GDPR (General Data Protection Regulation) requirements for data privacy and security, OpenApp have developed a key feature that means patients’ personal identifying information is stored separately from the survey data provided by the user, this is done to increase security around identifiable information. The platform also comes with a consent model that collects dynamic, multi-layered and explicit consent from patients, with an accompanying consent audit trial.
Communications – OpenApp configured our communications module tailored to Children Tumor Foundation’s requirements, this allows communication between the patients and researchers via email, if the patient consents to be contacted regarding any upcoming clinical trials/studies related to NF. The communications module can filter patients so only those that align with the specific set of research criteria are contacted.
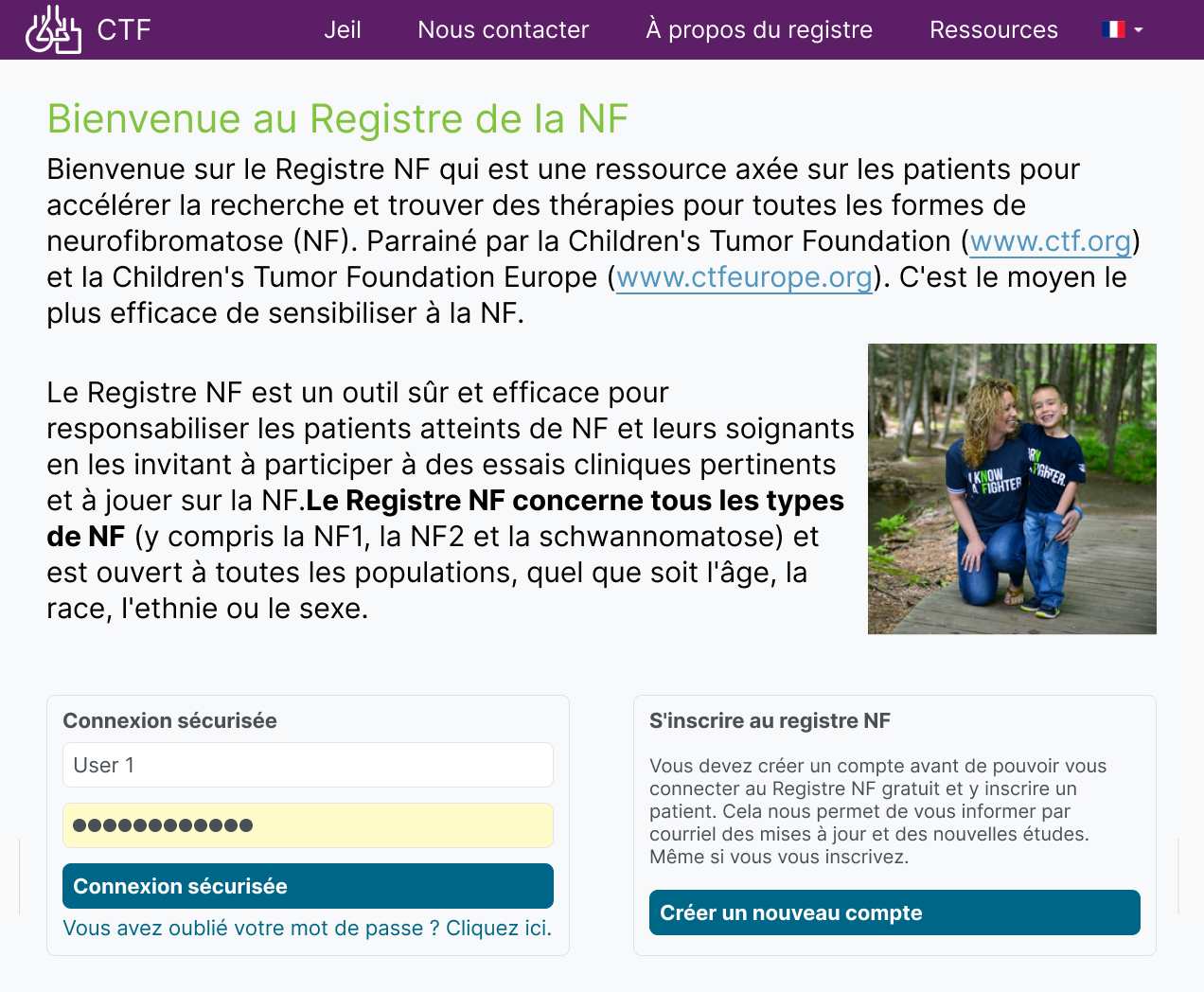
Multilingual – OpenApp have developed a registry that supports multiple languages. Presently, the platform supports English, French, Spanish, Portuguese and Italian with scope to add languages as they are needed.
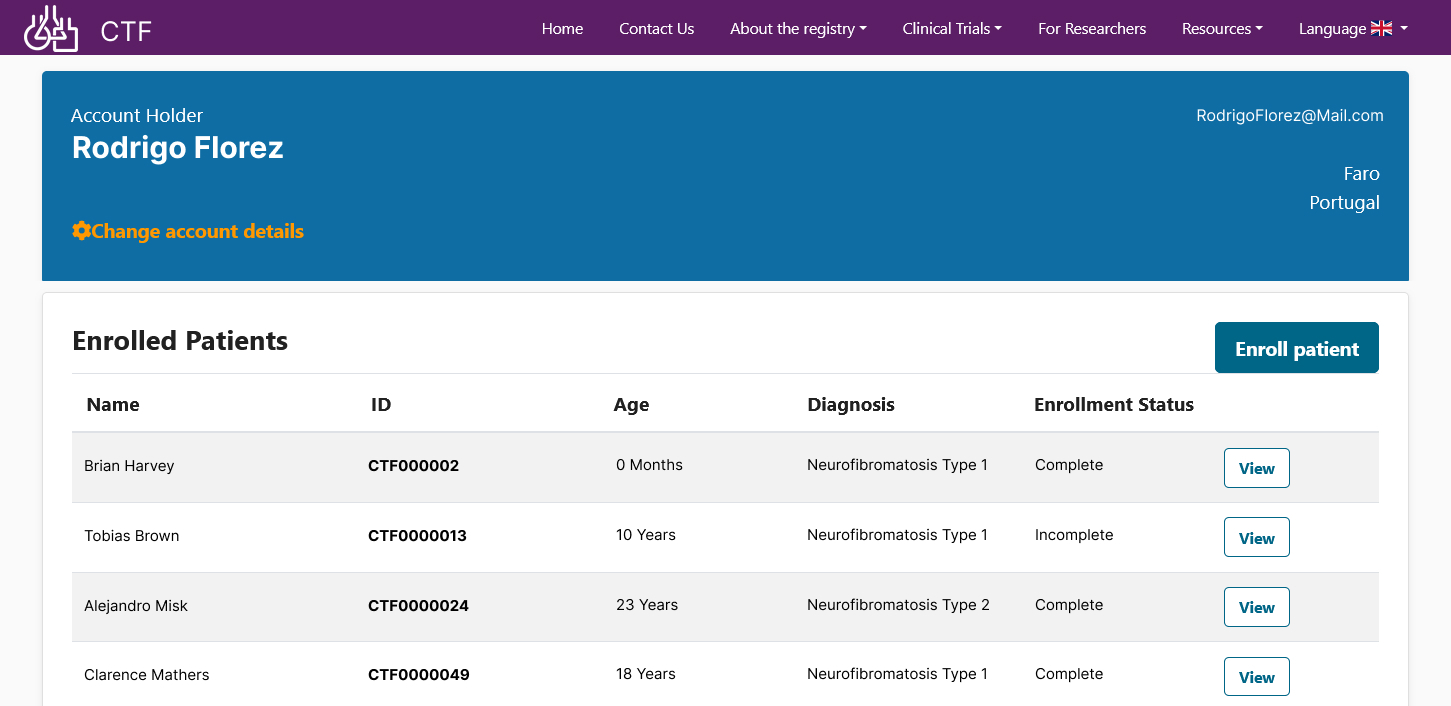
Automated Curation – With automated curation against pre-defined acceptance criteria, outlined by Children's Tumour Foundation, NF patients can self-enroll in a streamlined fashion and do not have to wait/or depend on clinicians or healthcare practitioners to enroll them. This allows Children's Tumor Foundation to continuously and effectively build their cohort across the NF global patient population.
Benchmarking – The registry is built to support benchmarking in real time, once a patient has completed a survey they can see how their answers compared to other NF patients across the world. These comparisons are broken down into easy to understand graphs and visualizations.
Results

hiOne registry for all NF types (NF1, NF2, and schwannomatosis): One platform to accommodate all NF patients. The system is open to all NF patients across any age, race, ethnicity, or gender. Understanding NF’s impact across all types of people from all over the world, from childhood to adulthood, is essential to finding the answers that will improve the lives of all NF patients. One disease specific registry is also important to reduce data fragmentation, meaning data can be benchmarked more easily and there is access to a larger cohort of NF patients to participate in important research.
Research: The collected data is anonymized so it can be used for research and other clinical trials/research objectives without any privacy violations. This data can be exported through excel, CSV and C-Disc.
Patient Engagement or (Patient-driven): Offers the patient or their representatives a space to be actively involved in reporting on their condition through user friendly forms and surveys. The platform can communicate to inform users of specific and appropriate research opportunities they can partake in, advancing clinicians and researchers understanding of NF.
Alerts and notifications: Patients will be alerted through the communications module, which will inform them about any upcoming research opportunities, if they qualify for, and have consented to be contacted regarding their potential participation.
Information Hub: The platform informs Patients about potential new treatments/studies for medical or general well-being with an NF focus that Children's Tumor Foundation deem relevant, and also offers information on Clinical Trials and their relevant phases. There is a space for NF news, such as care/clinical guidelines and medication approvals, and access to opinions of key thought leaders in the NF world.
User Friendly: The platform has been designed and developed with end users in mind, making it an easy to use system with a user-friendly intuitive interface and dashboards. This is essential as it makes the system simple to navigate and use, encouraging engagement and sustainability.
To read more about the registry in Children's Tumor Foundation's words we encourage you to visit these resources: understanding the NF registry and NF registry welcome page
For more information on our patient registry software, please visit: OpenApp patient registry software page .
To find out more about our custom software solutions, or to talk to us about similar work you may undertake, contact us here.
Latest News
Sorry, we couldn't find any posts. Please try a different search.
IQVIA (NYSE:IQV) is a leading global provider of advanced analytics, technology solutions and contract research services to the life sciences industry dedicated to delivering actionable insights. Learn more at www.iqvia.com.

Copyright © 2024 | Privacy Policy | Information Security Policy
OpenApplications Consulting Ltd. Registered in Ireland No. 355595

