Clinically Reported Data Patient Registry Platform
An intuitive, easy-to-use product that allows clinicians to enter, view and manage pertinent patient data longitudinally. This can be the distinct data gathering approach with your registry or can be combined with patient reported data, to learn more, please visit: Patient Reported Data.
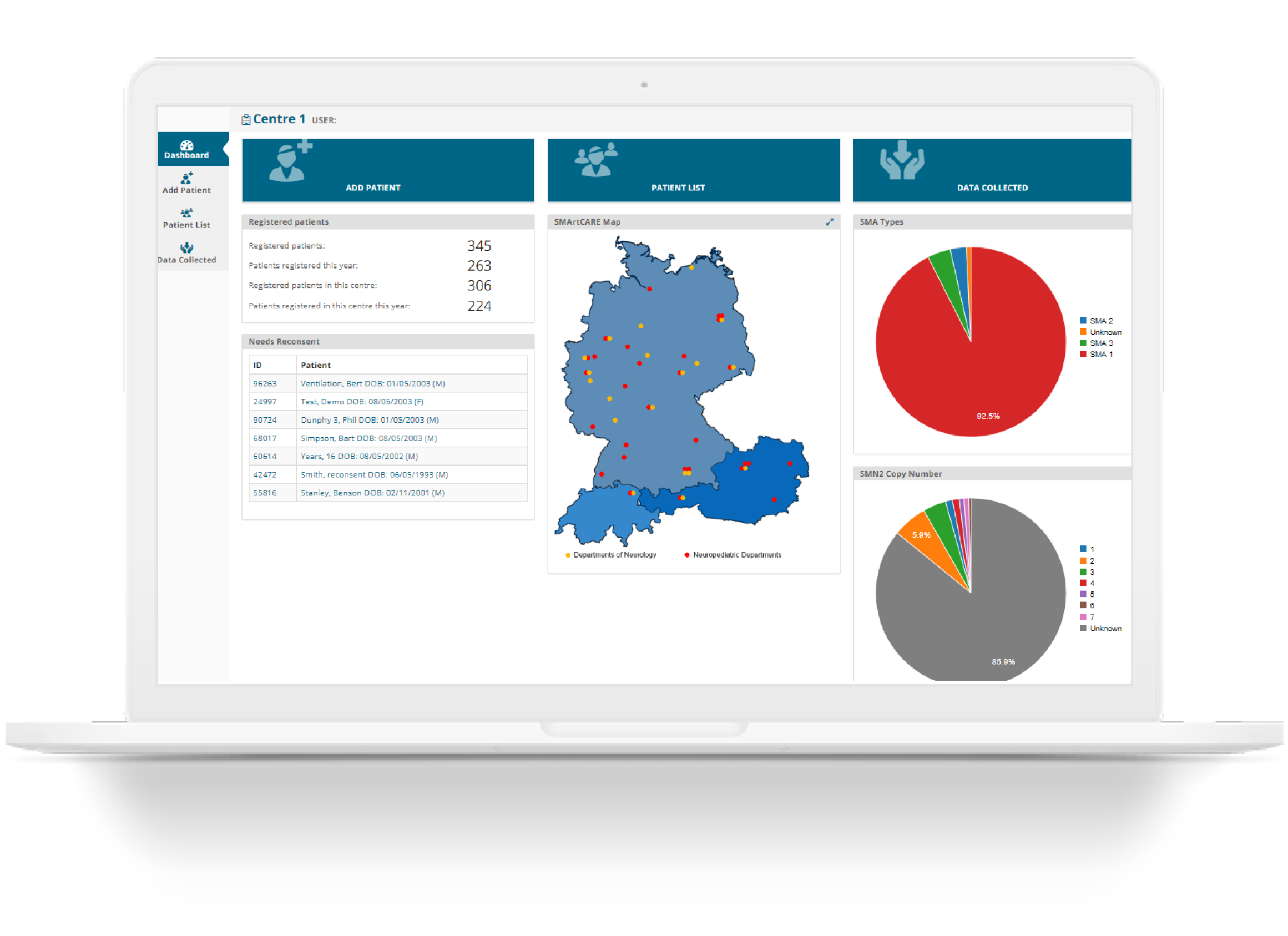
Intuitively manage longitudinal and study-specific real-world data collection in clinical settings.
Enable your clinical researchers to easily see the status of a patient or the entire research dataset.
Easily see overall status, trends and changes with data visualization based on all the data collected or specific subset of interest.
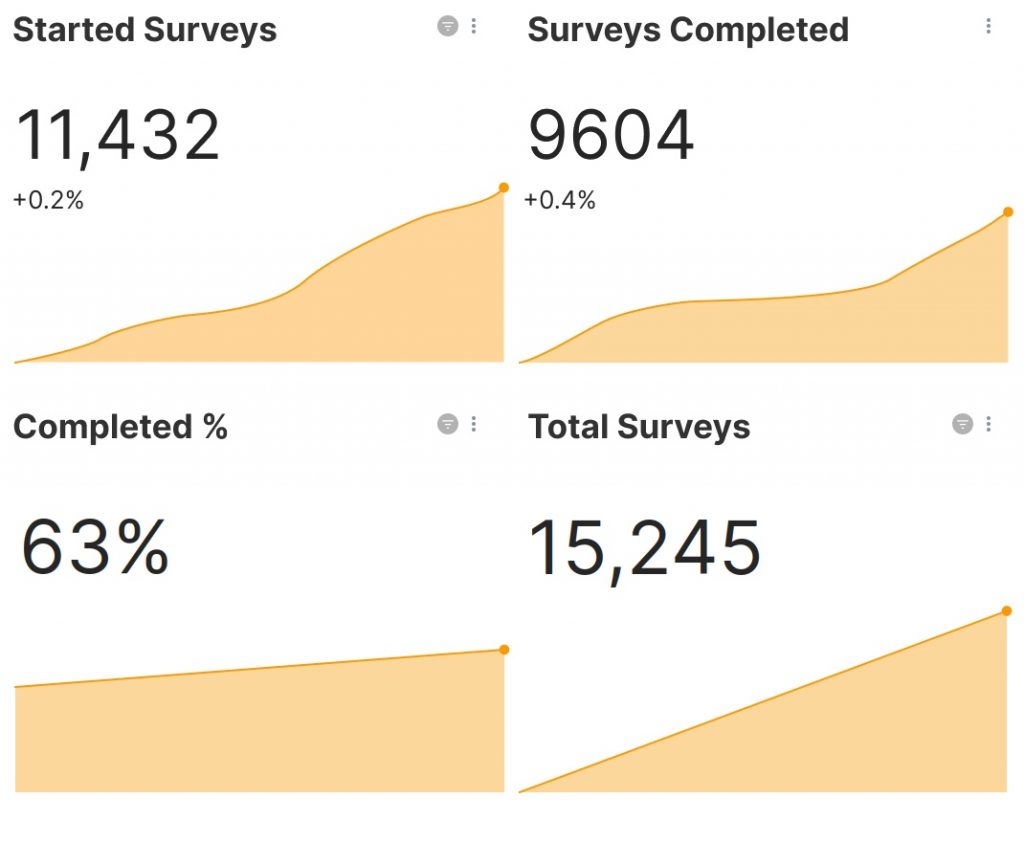
- Provide insights on a specific condition by collecting and consolidating data from a group of people with that condition.
- Visualise data important to the patient daily experience or center level using graphs, charts, pie charts about specific predetermined data points.
- See research data for an individual patient e.g. patient outcomes chart
Filter data based on all available data, or a specific cohort.
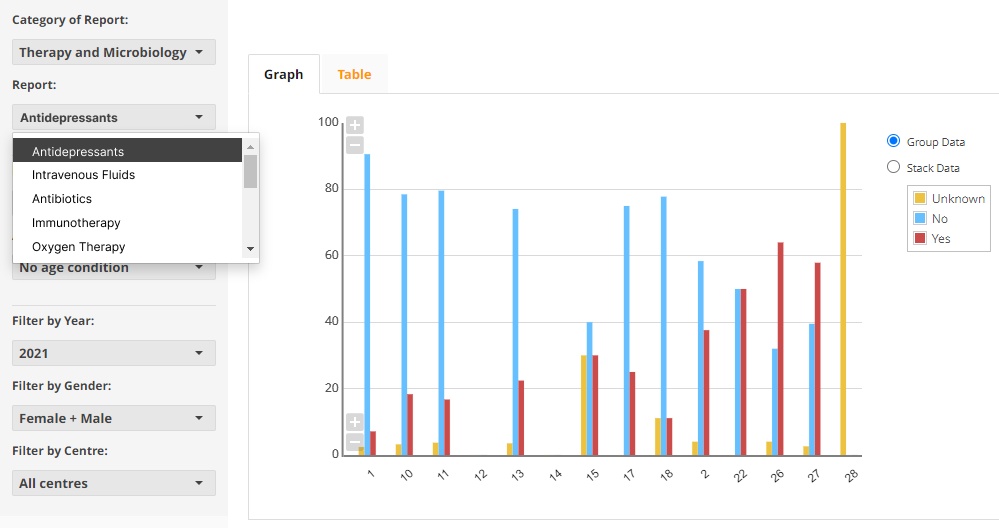
Compare and explore changes in an individual patient's disease experience with insightful visuals.
- Identify trends in patient experience by providing graphs/benchmarking against condition averages.
- Show patient's progress overtime by visually representing the collected data in a chart.
Study management reports provide summary of the study or registry data set.
- Provide information about the number of participants, or volume of data collected. For example by clinical center.
- Track the number of participants enrolled by number or list.
- Track number and status of open data queries.
Incorporate second-party data discrepancy management for complete, accurate and consistent data.

- Compare and explore changes in an individual patient's disease experience with insightful visuals.
- Provide information about number of registry participants.
- Indicate the volume of data collected, for example by clinical center.
- Show level of data completeness through a red-amber-green form status.
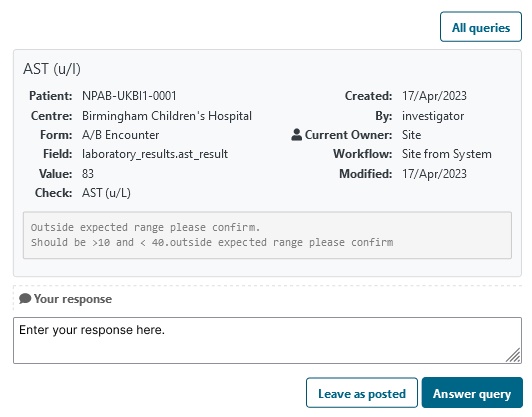
Manage data quality by verifying source data.
Communicate with your clinical data collectors by emailing outstanding and overdue actions.
- Define 'edit check' to establish valid versus invalid data standards within your protocol.
- Raise a flag against questionable data by checking the flag box and typing in a question.
- Prompt the data collector to review flagged data points by showing a list of raised questions.
- Enable Source Data Verification (SDV) for example against hospital data system or clinical charts.
- Email data collectors to make them aware of data gaps.
- Identify inconsistent data - query individual data discrepancies.
Integrate real-world data collection into clinical settings.
Integrate data collection into clinicians workflow.
Present eForms and electronic case report forms specific to the situation.
- Create scheduled sequences of case report forms (Visit 1, Visit 2 etc.)
- Include standardized scripts, to elicit the desired information, and instructions to guide usage and data-gathering.
- Provide standard templates / case report forms for common situation e.g. pregnancy.
- Set data collection frequency and parameters.
- Present conditional follow-up case report forms e.g. hospitilzations, adverse drug reactions.
Ensure data quality across clinical teams and sites by enabling multidisciplinary teams to collaborate.
- Show visualizations that enable clinicians to see how care regimes are affecting patient wellbeing , disease evolution.
- Collect data across multiple clinical sites while maintain confidentially by site.
Enable clinical data collectors to see their outstanding and upcoming data collection activities at a glance.
- Filter by patient visit / appointment.
- Show outstanding actions.
- Display individual patients status.
Aid clinician appointment planning by showing outstanding and upcoming data collection items.
- Present situation specific upcoming tasks based previous visits, information and study workflow.
- Facilitate visit planning by prompting suitable forms to fill in based on age and other previously entered data.
IQVIA (NYSE:IQV) is a leading global provider of advanced analytics, technology solutions and contract research services to the life sciences industry dedicated to delivering actionable insights. Learn more at www.iqvia.com.

Copyright © 2024 | Privacy Policy | Information Security Policy
OpenApplications Consulting Ltd. Registered in Ireland No. 355595

